
Written by: Mallory Gaines | February 23, 2022
African swine fever (ASF) is top of mind for industry members and government officials alike since its confirmation in the Dominican Republic last July. For the first time in a long time, the highly contagious swine disease is in our hemisphere and although the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) had already been preparing for a “what if” ASF outbreak situation, efforts have significantly increased, and all stakeholders are engaged.
An outbreak will mean many things for our industry, but in this blog, I am focusing on trade – specifically the APHIS Veterinary Services 16-4 form. IF YOU EXPORT ANY ANIMAL-BASED ANIMAL FEED OR PET FOOD (or sell to a customer that exports) YOU SHOULD KEEP READING!
You may be asking yourself, “So, what is the deal with the VS 16-4 and if I do export animal-based feed, with or without a porcine ingredient, why should I care?” Let’s examine the VS 16-4 form below.
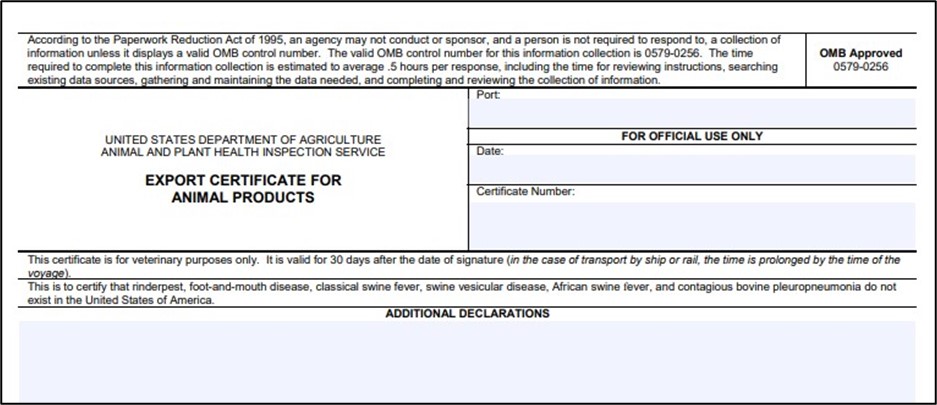
The language states that “rinderpest, foot-and-mouth disease, classical swine fever, swine vesicular disease, African swine fever, and contagious bovine pleuropneumonia do not exist in the United States of America.”
Almost all animal-based feed products and pet food and treats are exported utilizing the VS 16-4, which has been negotiated into existing export protocols. In the event of an ASF outbreak, the circled disease attestation above becomes invalid because the U.S. can no longer claim ASF “does not exist” in the United States.
APHIS spends countless hours negotiating with individual countries, agreeing to health measures and acceptable certificates, so manufacturers can export their products. If the VS 16-4 becomes invalid, APHIS will have to re-negotiate on a country-by-country, product-by-product basis, until a protocol for the U.S. to export these products is accepted and trade can resume.
Essentially, regardless of the animal-origin ingredient (including aquaculture) in your product, all animal food manufacturers will be barred from exporting their products in the event of an outbreak on the continental U.S. due to the invalidity of the 16-4 health certificate.
Even if you do not manufacture an animal-based product yourself, but your customer does, this will impact your business!
The American Feed Industry Association, along with several of our association partners, are engaging with APHIS, the Foreign Agricultural Service and the U.S. Trade Representative. The AFIA is asking APHIS to act now and begin the re-negotiation process with our trading partners today while we are not experiencing an outbreak.
The VS 16-4 concern is a top priority for the AFIA, and we will not stop pushing APHIS to be proactive on behalf of our members. While APHIS has already gone through the arduous government process and received approval to amend the VS 16-4, the language on the certificate has yet to be amended.
But we question the need or utility of the VS 16-4 altogether. What use does a veterinary certificate serve if it merely restates what the U.S. (and other countries) are already required to do, which is to report their specific animal disease statuses to the World Organization for Animal Health (OIE)? All the diseases, including ASF, on the VS 16-4 are OIE reportable diseases. So, knowing this, I ask you: VS 16-4, what is it good for?
Next time you speak with your local APHIS Service Center, ask them about this and what APHIS is doing to address this problem. Speak to those within your company about what this would mean for your business. Help us understand what this would mean for your business, your facilities and your employees by contacting Mallory Gaines, AFIA’s director of market access and trade policy, or commenting below.
Comments See our policy on comments